
- Home
- India
- World
- Premium
- THE FEDERAL SPECIAL
- Analysis
- States
- Perspective
- Videos
- Sports
- Education
- Entertainment
- Elections
- Features
- Health
- Business
- Series
- In memoriam: Sheikh Mujibur Rahman
- Bishnoi's Men
- NEET TANGLE
- Economy Series
- Earth Day
- Kashmir’s Frozen Turbulence
- India@75
- The legend of Ramjanmabhoomi
- Liberalisation@30
- How to tame a dragon
- Celebrating biodiversity
- Farm Matters
- 50 days of solitude
- Bringing Migrants Home
- Budget 2020
- Jharkhand Votes
- The Federal Investigates
- The Federal Impact
- Vanishing Sand
- Gandhi @ 150
- Andhra Today
- Field report
- Operation Gulmarg
- Pandemic @1 Mn in India
- The Federal Year-End
- The Zero Year
- Science
- Brand studio
- Newsletter
- Elections 2024
- Events
What are ‘super-sponge’ MOFs — compared to Hermione’s magical handbag, won 2025 Nobel Prize in chemistry for creators
Metal-organic frameworks (or MOFs) can absorb carbon dioxide and water vapour from air and toxic chemicals from drinking water like a sponge soaks up spilled liquids. As a magic spell allowed Hermione's handbag to hold vast supplies inside a seemingly small exterior in the Harry Potter fantasy, a few grams of MOF-5 have a surface area larger than a football field.

The Nobel Prize in chemistry for 2025 has been announced for Susumu Kitagawa from Japan’s Kyoto University, Richard Robson from Melbourne University in Australia, and Omar M. Yaghi from the University of California, Berkeley in the United States, for discovering the chemical technique to design and produce metal-organic frameworks (MOFs) that have the remarkable ability to absorb carbon...
The Nobel Prize in chemistry for 2025 has been announced for Susumu Kitagawa from Japan’s Kyoto University, Richard Robson from Melbourne University in Australia, and Omar M. Yaghi from the University of California, Berkeley in the United States, for discovering the chemical technique to design and produce metal-organic frameworks (MOFs) that have the remarkable ability to absorb carbon dioxide and water vapour from the air, as well as dangerous toxic chemicals like polyfluoroalkyl substances (PFAS) from drinking water. Much like a sponge that absorbs spilled liquids such as sambar, rasam or coffee on the kitchen counter.
"This year's laureates … have found ways to create materials, entirely novel materials, with large cavities on their insides," said Nobel committee chair Heiner Linke, a nanophysicist at Lund University in Sweden, at a press conference announcing the prize on October 8. "A small amount of such material can almost be like Hermione's handbag in Harry Potter — it can store huge amounts of gas in a tiny volume", he added.
In the Harry Potter fantasy series, authored by JK Rowling, with the magic spell of 'Undetectable Extension Charm’, Hermione's purple, beaded handbag could hold a vast amount of supplies inside a seemingly normal, small exterior. Like the magical purse, a few grams of MOF-5, about the size of a sugar cube and filled with micropores, has a surface area larger than a football field. It can hold nearly 0.107 g of CO2 per gram of MOF-5 at room temperature and pressure. The MOF DUT-60, designed in 2018, has a vast internal surface area of 7,839 square metres per gramme, which is roughly equivalent to six Olympic-sized swimming pools.
Also read: How rare interstellar comet 3I/ATLAS is whizzing through our solar system
Interestingly, Yaghi, one of the three awardees, was born to a Palestinian couple who were among the thousands forced to flee from the hamlet of Al-Masmiyya, near Gaza, in 1948 by Zionist militias through various violent means. Yaghi was born at Amman, Jordon at the Palastenian refugee camp.
Despite living on subsistence farming and in a single room shack with no electricity or running water in the refugee camp, Yaghi's barely educated parents insisted on educating their children. Yaghi travelled to the United States alone at the age of 15, urged by his father to pursue an education overseas. He knew very little English and was armed only with determination. He started at Hudson Valley Community College before transferring to the State University of New York at Albany, where he earned his bachelor's degree. He received his PhD in chemistry from the University of Illinois at Urbana-Champaign in 1990 and then pursued postdoctoral research at Harvard University.

Omar M. Yaghi. Photo: Brittany Hosea-Small, UC Berkeley; courtesy: nobelprize.org
In an interview with the Nobel committee's media team, Yaghi credited the U.S. public school system for his success. It "takes people like me with a major disadvantaged background, a refugee background, and allows you to work, and work hard and distinguish yourself", he said.
In the refugee camp in Amman, Yaghi used to get up early to fill a few buckets of water while the taps ran once in 14 days; now he has created an MOF that traps water molecules from the air's moisture, providing clean drinking water even in arid places. In a sweet revenge, he developed MOF-303, which can suck in water vapour even at low humidity. When the temperature dips in the desert at night, the material releases the trapped water, which can be collected and used as clean drinking water.
From waiting for water to inventing ways to pull it from air, Yaghi has come a long way, an inspiration for all. “My parents could barely read or write. It’s been quite a journey, science allows you to do it” Yaghi said.
If we look closely at a sponge, we will notice a structure that resembles tiny tunnels crisscrossing through it. When you compress the sponge, the air in its micropores escapes. These holes absorb liquids, such as water, via capillary action. Likewise, metal-organic frameworks have nano-scale microstructures with cavities.
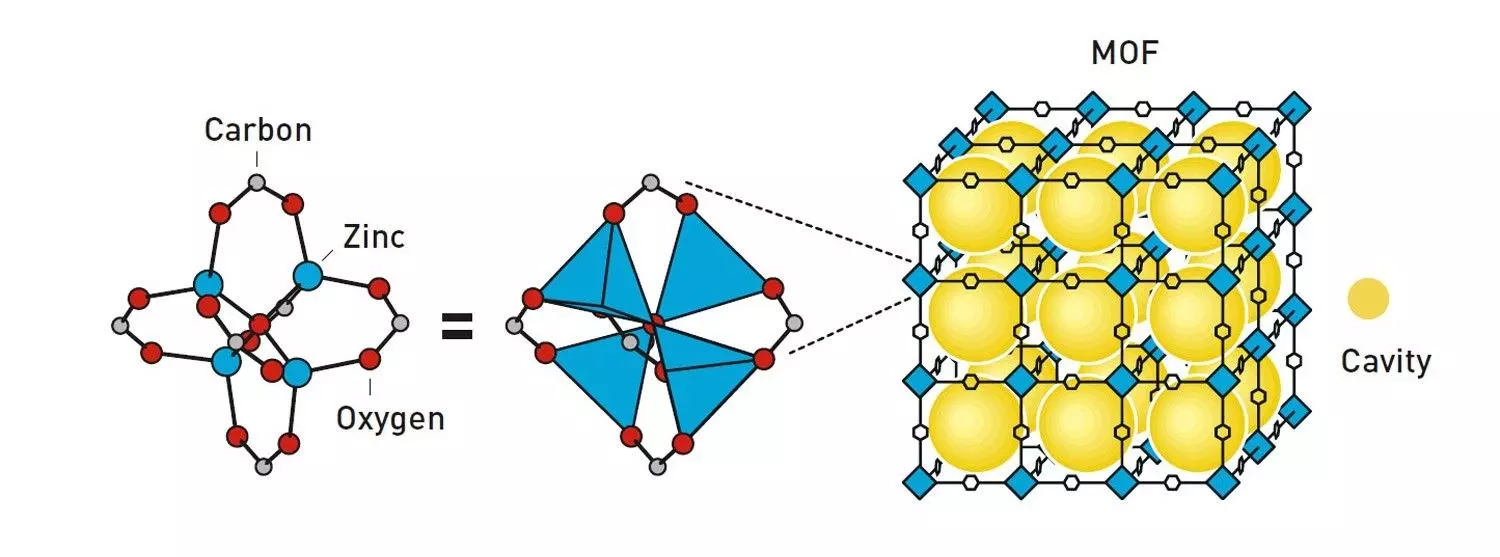
In 1999, Omar M. Yaghi constructed a very stable material, MOF-5, which has cubic spaces. Just a couple of grams can hold an area as big as a football pitch. Illustration: Johan Jarnestad/The Royal Swedish Academy of Sciences, courtesy nobelprize.org
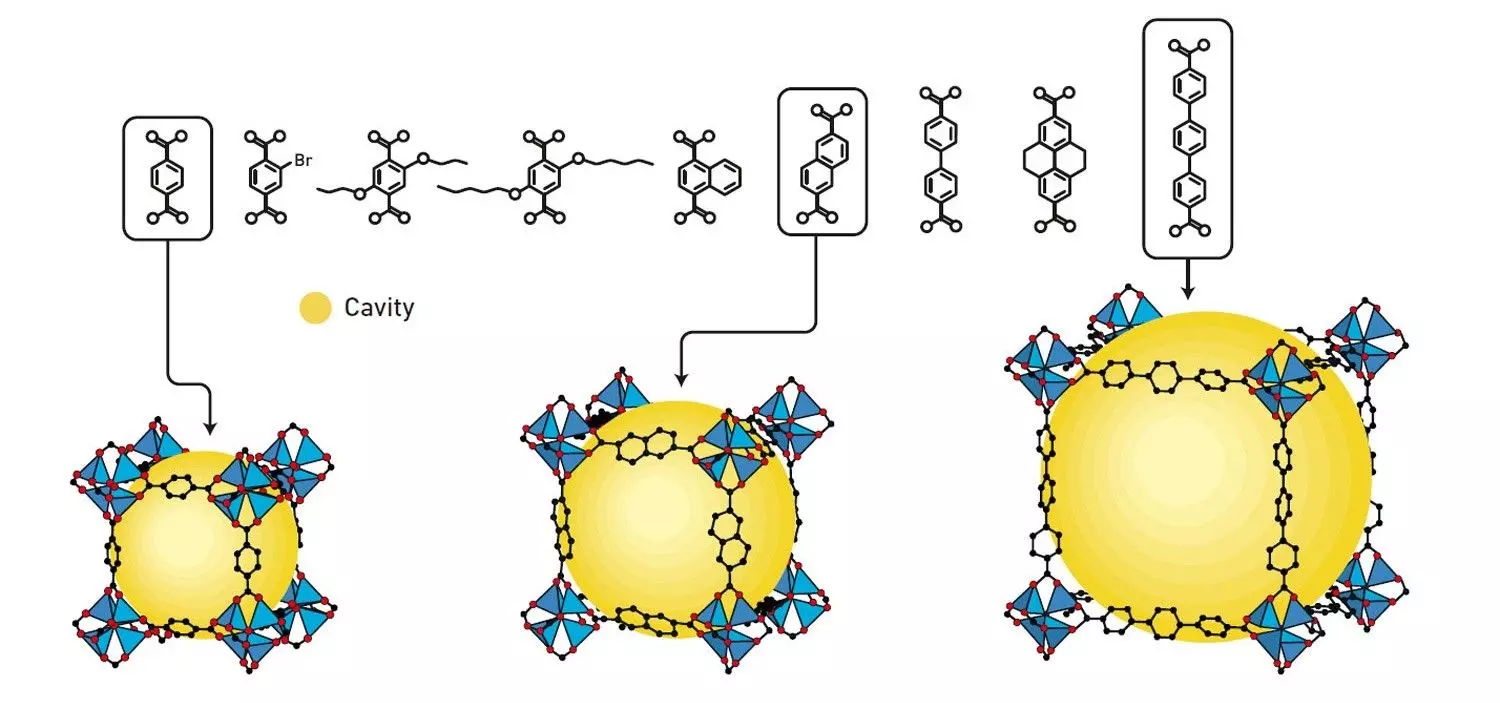
In the early 2000s, Omar M. Yaghi showed that it is possible to produce entire families of MOF materials. He varied the molecular links, which resulted in materials with different properties. These include 16 variants of MOF-5, with cavities of various size. Illustration: Johan Jarnestad/The Royal Swedish Academy of Sciences, courtesy nobelprize.org
MOFs are porous polymers composed of metal ion clusters at the junctions and organic ligands at the edges, resulting in one-, two-, or three-dimensional structures. Metal-organic frameworks are formed much like bamboo sticks are connected to form a hut's frame. While the organic molecule clusters act as bamboo sticks, the metal ions aid in binding them together, creating a 'cage'-like structure with a hollow micro cavity. The MOF supermolecules arrange themselves into a precise crystalline structure, similar to how the carbon in a diamond forms a lattice. This supermolecular crystal, like a sponge, contains numerous micropores. These materials are said to transform how we use materials in the future.
Also read: How astronomers spotted birth of a new solar system around baby star HOPS-315
Surface area is key in diverse chemical reactions. It determines the rate of reaction, for example, in dissolving. If we put a lump of jaggery directly into water, it takes longer to dissolve. To dissolve it quickly, we need to break the lump into smaller granules. When it is in lump form, its surface area is small. The dissolution reaction occurs only on that surface where water interacts. Only after one layer of jaggery dissolves does the underlying layer come into contact with water. If you break it into granules, each small grain has its own surface area, which is indeed very small. However, with hundreds and thousands of grains, like small drops making a flood, the total surface area swells. That is why granules dissolve faster than lumps.
Absorption reactions, like dissolution, depend on surface area. Therefore, with just a few grams of metal-organic frameworks like MOF-5, large quantities of substances like carbon dioxide can be stored.

Susumu Kitagawa. Photo: Japan Academy, via Wikimedia Commons; courtesy nobelprize.org
The number of cars and two-wheelers that can be parked in a bazaar ground depends on its surface area. If the population increases and the number of vehicles grows, that space becomes insufficient. Therefore, to provide additional parking, we built multilevel car parks with multiple floors stacked one on top of the other.
The MOFs are like multilevel parking, or a multistory hotel. The cavities on the inside are "almost like rooms in a hotel, so that guest molecules can enter and also exit again from the same material", said Linke during the Nobel award announcement. Reticular chemistry that deals with linking molecular building blocks into extended crystalline structures, the MOFs, is like designing multilevel parkings: right parking slots for various types of vehicles —cars, two-wheelers, and heavy vehicles. By using the appropriate organic molecules, the cavity size can be increased or decreased. With the right-sized cavity and the presence of appropriate metal ions and organic molecules, the desired type of gas molecules can be trapped.
Also read: Why Earth is in a hurry and July 9 saw one of the shortest days ever
In the late 1980s, Robson discovered that bonding copper ions to carbon-based compounds yields pyramid-like structures similar to the tetrahedral carbon lattice in a diamond. He also found that these tetrahedral MOF supermolecules arrange themselves in an orderly fashion to form crystals. A diamond is a very dense, sturdy material; it does not even scratch easily. However, despite its diamond-like shape, the novel copper-organic framework, MOF, was found to be porous. This was the first ever MOF created. But the MOF did not last long; it degraded rapidly, making it unusable for any practical purpose.
A few years later, Kitagawa and his colleagues began designing new metal-organic framework materials. They created materials capable of absorbing and storing gases such as methane, nitrogen, and oxygen. Around the same period, Yaghi conducted experiments bonding metal ions with organic molecules. Around 1999, he created a remarkable material called MOF-5 by bonding zinc oxide metal molecules in a tetrahedral arrangement to the organic molecule benzenedicarboxylate. This MOF was able to store hydrogen in its pores and subsequently release it.
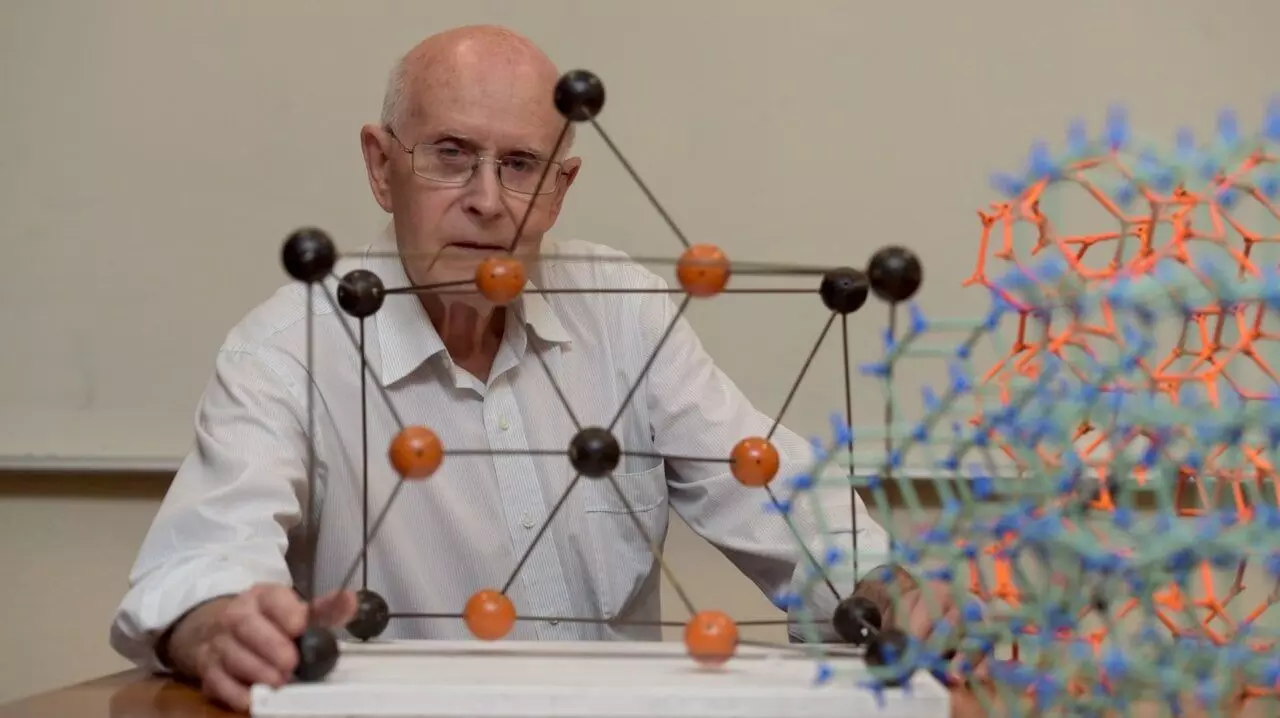
Richard Robson. Photo: Paul Burston/University of Melbourne; courtesy: nobelprize.org
Yaghi's 1990s work defied conventional chemistry by creating stable crystalline structures capable of trapping gases, filtering toxins, and storing energy. These metal-organic frameworks have since inspired a variety of uses, including sustainable energy storage and water generation in arid places. Yaghi also designed chemical processes to produce MOFs in large quantities for practical applications.
While MOFs are designed and built artificially, several molecules in nature, from haemoglobin, which carries oxygen to various parts of the body, to chlorophyll, the heart of photosynthesis, resemble metal-organic frameworks.
A sponge absorbs all kinds of liquids without distinction, whether coffee or rasam. However, like the mythical swan, Hamsa or Annapakshi, that separates milk from a mixture of water and milk and drinks only the milk, metal-organic frameworks can be designed to selectively absorb specific chemical substances from a mixture, depending on the metal atoms and organic molecules used.
Certain atoms bond only with specific atoms. Similarly, certain molecules can form chemical bonds only with particular types of molecules. Using this chemical understanding, chemists have developed various metal-organic frameworks for diverse applications over the past few decades.
Also read: The DNA of India: How Neanderthal genes, ancient migrations, and caste shaped a billion people
Since the 1980s, when the first MOF was created, approximately one lakh varieties of MOFs have been designed and developed for storing gases, acting as catalysts, trapping hazardous chemicals in water, and transporting pharmaceuticals, among many other applications.
The recently developed zinc-based MOF, CALF-20, traps CO2 molecules in its pores, even in the presence of water. Clinical trials of the MOF RiMO-301 are underway for cancer radiation therapy. The RiMO-301 is injected into tumours before low-dose radiation therapy, and preliminary results indicate 42 per cent of people who otherwise would not have responded to radiation therapy responded to RiMO-301. MOFs like UiO-66 and MIL-53(Al) are effective at adsorbing toxic PFAS.
In India, too, several research institutes are involved in the design and development of novel MOFs. The group led by R Vaidhyanathan of the Indian Institute of Science Education and Research (IISER), Pune, developed Nickel Isonicotinate MOF, labelled IISERP-MOF2, which can serve as a CO2 capture sponge, even in the presence of moisture from moist coal, and made news. The group led by Sanjay Mandal at IISER Mohali, recently published research on a highly emissive fluorescent zinc-MOF for sensing applications.
